Our Research
Our Research Philosophy
Team Hydro’s approach to funding research grants is simple:
- We support research with the potential to move science towards a cure for Hydrocephalus, rather than just small variations on the clinical status quo. This includes projects that seek to uncover the basic biology underlying this disease, to determine and halt causative factors, and to generate innovative new approaches to therapy.
- We support promising researchers with potential for long-term impact on the field. The lifeblood of academic research in the U.S. is, generally speaking, large federal grants issued via the NIH, the DoD, and other public research agencies. We have no intention to replace these agencies! However, it is currently impossible for new researchers (especially those interested in under-funded conditions like Hydrocephalus) to qualify for lab-sustaining grants from these departments without preliminary data. By the same token, commercial R&D typically enters the foray only after preliminary data developed via private and public investment. At Team Hydro, we seek to provide hydrocephalus researchers with seed funding that will enable them to assemble the critical mass of data necessary to bridge the gap towards the major public grants (and/or commercial development). In doing so, we hope to help their labs to become self-sustaining entities for research, discovery, and the training of new talent that will continue in the field for many years to come.
To this end, Team Hydro has raised nearly $2,000,000 for hydrocephalus research and supported a range of research grants in the U.S., Australia, Greece, and Canada. Importantly, all our grantees are selected as a part of rigorous peer review process in conjunction with expert panels at the Hydrocephalus Association. Team Hydro has a policy of 0% indirect costs on all our grants, which has been in place for the funding of all of these grants.
We at Team Hydro have been extremely pleased with the results of this investment model so far. In fact, we have already seen more than $16 Million in follow-up grants to Team Hydro labs through the NIH and Department of Defense. See below for descriptions of the projects we’ve funded to date.
If you are a researcher and would like to apply to receive funding, please first check out the grants page at the hydrocephalus association to see if there is an active RFA (we typically fund research projects selected through these grants). If you have high-impact work that you do not think fits with any active RFAs, feel free to contact us directly at info@teamhydro.org.
Our Grants
At Team Hydro, our research portfolio is designed to foster a diverse arsenal of novel tools to prevent and treat hydrocephalus. To understand this portfolio, it helps to understand a bit about what causes hydrocephalus:
Background: Hydrocephalus and its Causes
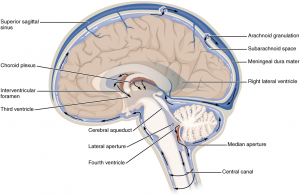
The brain and spinal cord are surrounded by cerebrospinal fluid (CSF), a clear liquid that cushions the brain and serves additional functions such as delivering hormones and clearing waste. CSF is produced near the center of the brain by special clumps of cells called choroid plexus, then flows through a network of cavities called the ventricular system, and finally wraps around the brain and spinal cord before being absorbed into the body’s blood vessels. In other words, we have:
Hydrocephalus, the accumulation of excess CSF fluid in the brain, is a deadly condition that can arise from an imbalance at any stage of the above process. Over-production of CSF is rare but can be caused, for example, by choroid plexus tumors that secret extra CSF. Obstructed CSF flow is extremely common, and can be due to brain bleeds (#1 cause), tumors, or any other injury that ultimately blocks the path of CSF through the brain. Failure of CSF reabsorption is typically thought to drive a condition called Normal Pressure Hydrocephalus and can develop with aging or due to damage to the reabsorption sites at any age by meningitis, surgery, radiation, or trauma. Regardless of the cause, hydrocephalus is deadly without treatment. Unfortunately, the only treatments are surgical in nature and have only a 50% chance of lasting even two years.
Towards a 21st-Century Hydrocephalus Toolkit
With the above in mind, our goal is to build up a rich arsenal of tools that can be used to tackle hydrocephalus from every angle. These include projects for:
- Decreasing CSF production through new drug mechanisms (Blazer-Yost via TRPV4; Lehtinen via Gene expression/protein secretion; Podvin via augurin)
- Preventing obstruction from developing after brain bleeds, by blocking (Strahle via Iron; Limbrick via ADAM10; Eskadardi via complement inhibition; McAllister via exosome inhibition)
- Reversing obstruction by reprogramming scar tissue into health brain cells (Taraviras lab)
- Preventing accumulated CSF excess from damaging the brain, by reducing neuroinflammation and resulting tissue stiffness (McAllister)
- Reabsorbing excess fluid by turning on alternative CSF exit pathways (Ding via glymphatics; Linninger via AQP4), and
We also fund research designed to overcome fundamental limitations in current therapies, to increase their chances of becoming lasting cures. This includes:
- Building a new “choroid plexus-on-a-chip” platform to accelerate testing of therapeutic compounds (Harris)
- Conducting multi-omic analysis of hydrocephalus patients to identify new target mechanisms and find new drugs (Haller)
- Elucidating ventricular shunt clogs to prevent shunt failure (Harris)
- Building new tools to deliver drugs into the brain, to facilitate all of the above (Yun Yung)
See below for more details on each of these grants.
More Details on Current and Former Projects Sponsored by Team Hydro
Histotripsy for the treatment of hydrocephalus — Sukovich Lab, University of Michigan
Summary from Applicant: Current management strategies for hydrocephalus rely on invasive surgeries including endoscopic third ventriculostomy (ETV), choroid plexus lesionectomy, and ventriculoperitoneal/pleural shunt replacement. Beyond inherent risk of such invasive procedures , there is also high risk of post procedure shunt failure leading to additional
surgeries and significant morbidity and mortality. There is no need for an incisionless, non-invasive approach for managing hydrocephalus. Histotripsy is a non-invasive, ultrasound-based ablation therapy that uses targeted aviation generation to mechanically fractionate and liquefy tissues. Histotripsy offers an opportunity to perform ETV, choroid plexus lesionectomy, and clearing ventriculostomy tubing without making an incision. In this proposal, the researchers will conduct experiments to demonstrate the feasibility and accuracy of using histotripsy to perform these procedures in a cadaveric model. Histotripsy has the potential to meet the need for incisionless, non invasive surgical treatments for hydrocephalus treatment through ETV, choroid plexus lesionectomy, and as an adjuvant therapy for maintaining shunt patency. Grant value: $50k
Cellular and Molecular Characterization of Human and Porcine Choroid Plexus to study Post-Hemorrhagic Hydrocephalus (PHH) — Lehtinen Lab, Boston Children’s Hospital
Bleeding into the cerebral ventricles is the leading cause of hydrocephalus in the United States, and interventions to treat this condition remain suboptimal. The Lehtinen laboratory and others have recently identified the choroid plexus (ChP) as a first responder to intraventricular blood and, therefore, a key contributor to the development of hydrocephalus. Despite major research advances utilizing rodent models of hydrocephalus, translation to the human clinic has historically been limited. This is likely due to our lack of understanding of the fundamental differences in the development of hydrocephalus between rodents and humans. The pig offers an intermediate species for preclinical testing of therapies, and here we propose to compare
human and pig ChP in the development of hydrocephalus following bleeding. We can then compare these data to our existing rodent data to elucidate the most promising mechanisms for developing novel therapies. Grant value: $50k
A human choroid plexus-on-a-chip to study cerebrospinal fluid secretion and pharmaceutical treatment of hydrocephalus — Harris Lab, Wayne State University
Why are surgical approaches still a mainstay of hydrocephalus treatment? Why isn’t there a pharmaceutical, non-surgical strategy to alleviating symptoms of hydrocephalus despite decades of research? The answer: the mechanisms driving cerebrospinal fluid (CSF) secretion through the choroid plexus (CP) are not completely understood. There is a critical need for a model that gives researchers unincumbered access to study how the CP functions. This research proposal will develop a tool to accelerate the investigation of mechanisms underlying the functions of the CP. The CP-on-a-chip developed in this project will then be used to test a hypothesis pertaining to how inflammation may trigger TRPV4 activation and lead to CSF hypersecretion at the CP. Understanding how the “secretion” and “barrier” functions of the CP are altered in response to inflammation will enable us to develop more effective pharmaceutical approaches to hopefully mitigate the symptoms of hydrocephalus without the need for surgeries! Grant value: $50k
Multiomics of Post-Hemorrhagic Hydrocephalus — Haller Lab, Washington University in St. Louis
Very few risk factors have yet been determined that contribute to risk of developing hydrocephalus following intracranial hemorrhage. Further, the functional mechanisms and downstream effects of hemorrhage leading to hydrocephalus have not been characterized. There is a need for novel, unbiased approaches to identify and describe the pathways that lead to PHH. This project will fund molecular profiling of tissues relevant to PHH (cerebrospinal fluid (CSF)) from clinically well-characterized hydrocephalus patients to 1) identify novel genes implicated in disease, 2) understand the biology of the disease, 3) identify gene-specific pathways leading to disease, 4) identify new potential biomarkers, and 5) nominate potential drugs that could be repurposed for PHH. To do this, the researchers will generate genomics, transcriptomics, proteomics, metabolomic and lipidomic data from two large, well-characterized hydrocephalus cohorts. Grant value: $50k
Complement inhibition in hydrocephalus therapy –Eskandari Lab, Medical University of South Carolina
Developing pharmacotherapy for hydrocephalus is hindered by its complex multifactorial nature and the current rampant dogma that surgery is the only option. Understanding the underlying of mechanisms of hydrocephalus-related brain injury can overcome this narrow view, opening doors for medical therapeutics to improve outcomes and even prevent or cure the condition. These researchers have collaboratively identified a complement-induced etiological component of post-hemorrhagic hydrocephalus (PHH). However, several physiological functions are associated with the complement system and prolonged systemic inhibition is detrimental. They have therefore developed a novel peripherally injected/site-targeted complement inhibitor (PSel-Crry 2.3), which reduced PHH, reduced brain injury volume and improved neurological function in a mouse model of germinal matrix hemorrhage (GMH). This project funds investigating the most efficacious dose and initial treatment window using the same GMH model. Successful completion will enhance the understanding of PHH and propel the development of therapeutic medication for this solely surgical condition. Grant value: $50k
Cerebrospinal Fluid Profiling in Infants with Hydrocephalus: Defining a Novel Pathophysiology Pathway — McAllister Lab, Washington University in St. Louis
No studies have identified the complete expression profile of cells and exosomes in the cerebrospinal fluid (CSF) in post-hemorrhagic hydrocephalus (PHH). The McAllister Lab’s preliminary results indicate that neural stem cells (NSC), oligodendrocyte-related progenitor cells, and immune cells are present in the CSF of PHH patients. They have also confirmed that exosomes carry pro-inflammatory cargo, such as S100A proteins, in the CSF in PHH. This project will test the central hypothesis that PHH pathogenesis and its associated developmental disabilities are mediated through the effect of CSF-based cellular and exosomal inflammatory signaling on the ventricular/subventricular zones (VZ/SVZ) by (1) defining the cell composition and gene-activated pathways in the CSF from neonates with PHH, (2) examining the role of exosomes in inflammatory and neurodevelopmental mechanisms in hydrocephalus, and (3) analyzing S100A9 in PHH as a potential therapeutic target. This work will provide new research directions into the pathophysiology of PHH and possible therapeutic targets. Grant value: $50k
Preclinical Testing of SGK1 Antagonists for the Treatment of Hydrocephalus– Blazer-Yost Lab, Indiana University – Purdue University Indianapolis
Though hydrocephalus results from a multitude of causes, both genetic and acquired, there are convergent pathophysiological mechanisms that can be pursued in the development of nonsurgical intervention strategies. Previous pharmacotherapies have largely failed in the clinical setting for the management of hydrocephalus. This is due to several reasons: (1) inappropriate target distribution leading to off-target deleterious effects, (2) failure to converge on multiple pathogenic mechanisms, and (3) lack of robust and adequately powered preclinical proof-of-mechanism studies. This project pursues a promising new target, inhibition of serum- and glucocorticoid-induced kinase 1 (SGK1), for the treatment of hydrocephalus. SGK1 inhibitors inhibit transepithelial ion transport and thus may reduce CSF production. This grant will support three experimental aims intentionally designed to provide a robust body of preclinical studies to advance the SGK1 inhibitor toward a clinical trial. These studies represent a novel target for the treatment of hydrocephalus using a proprietary compound that is a specific inhibitor of SGK1. Grant value: $50k (Note: See here for our most recent interview with Dr. Blazer-Yost about the recent $11.3M follow-on funding of her work from the Department of Defense!)
Reprogramming scar tissue into healthy ependymal cells — Taraviras Lab, University of Patras, Greece
Human hydrocephalus is characterized by an abnormal circulation/accumulation of cerebrospinal fluid in the brain ventricles. Loss and/or dysfunction of ependymal cells has been linked to hydrocephalus formation in mice and humans. Current treatment of hydrocephalus only partially relieves the symptoms leaving the disease basically untreated. Recent findings identify GemC1/Lynkeas and McIdas, as central regulators of the ependymal cell fate commitment and differentiation, while mutations in GemC1/Lynkeas and McIdas have been associated with hydrocephalus in humans. The goal of this project is to provide evidence for novel directions on hydrocephalus treatment. The researchers will assess the ability of GemC1/Lynkeas and McIdas to induce direct cellular reprogramming towards the ependymal lineage, of cells lining the wall of hydrocephalic mouse models. Towards this direction a genetic mouse model of hydrocephalus established in our laboratory and a mouse model of intracranial hemorrhage will be used. Grant value: $50k
Gene expression in the tissue surrounding the shunt catheter — Harris Lab, Wayne State University
Current understanding of the cellular response contributing to the failure of cerebral spinal fluid (CSF) shunts has been limited, to date, with the evaluation of cells and tissue around the implant. Yet there is a critical knowledge gap in gene expression profiles in this tissue. When paired with cellular analysis, gene expression can lead to understanding the activity of the cells obstructing catheters at the time of shunt failure. This first-time study, which pulls strengths from the recent world-renowned Barres et al. study on astrocyte activation, represents a robust investigation of the changes in gene expression levels specific to astrocyte immune response following CSF shunt implantation. By shedding light on the mystery of astrocyte phenotype expression on shunt surfaces, root causes for shunt failure can be achieved. This Innovator Award will drive urgent investigation into manipulation of astrocyte activity using shunt modification or drug release. Grant value: $25k.
Iron-mediated ventricular injury in posthemorrhagic hydrocephalus — Strahle Lab, Washington University
Previously, this group showed that iron play a key role in the development of hydrocephalus and neuronal cell death after intraventricular hemorrhage. It is not known, however, how iron enters into ependymal choroid plexus cells in the brain to cause this damage. This project will determine the mechanism of iron entry into the ependymal and choroid plexus cells, which will allow for the development of directed treatments to inhibit cellular entry of iron, preventing neuronal damage and hydrocephalus. Grant value: $50k. (Note: This investment resulted in a $2.4M dollar follow-up grant form the NIH! See here for an interview with Dr. Strahle!)
Preclinical Testing of TRPV4 Antagonists for the Treatment of Hydrocephalus– Blazer-Yost Lab, Indiana University – Purdue University Indianapolis
Our preliminary data indicate that TRPV4 antagonists ameliorate hydrocephalus in a rat model of Meckel Gruber Syndrome. The goals of the proposal are to: 1) determine if the efficacy is a class action of TRPV4 antagonists; 2) determine if TRPV4 antagonists are effective in another model of hydrocephalus representing a different species and different genetic mutation and; 3) use a continuous choroid plexus cell line to examine the effect of TPRV4 agonists and antagonists on transepithelial ion flux. Drug treatment will be followed with state-of-the-art rodent MRI for quantification of ventricular volumes. The cell line will be studied using well characterized electrophysiological techniques. If successful, these studies will form the framework for future pharmacokinetic and pharmacodynamic testing of TRPV4 antagonists, and structural/functional studies linking changes in brain metabolism with behavioral changes. The proposed studies will make a substantial contribution to progression toward developing the first drug treatment for hydrocephalus. Grant value: $50k.
Goal: Prove the efficacy of a compound to ameliorate ventriculomegaly in multiple mouse models. (Note: This grant resulted in a $1.3 million follow-up grant from the DOD in 2017. See here for a Q&A with Dr. Blazer-Yost! — and another $11.3 in 2023!)
Impact of germinal matrix hemorrhage on CSF reabsorption through the glymphatic system — Yan Ding, Loma Linda University
Working at the John H. Zhang’s laboratory at Loma Linda, Dr. Yan Ding is doing pioneering work investigating the role(s) of the brain glymphatic and lymphatic systems in CSF absorption and the development of PHH. These systems, of great interest over the last few years but under-explored to date, may hold therapeutic potential in PHH and other forms of hydrocephalus. Grant value: $50k. (Note: Yan Ding and John Zhang from Loma Linda have received a $1.7M grant from the NIH to study hydrocephalus. See here for an interview with Dr. Ding!)
Pharmacological Prevention of PHH of prematurity — David Limbrick, Washington University in St. Louis
Ventricular zone (VZ) disruption is a fundamental step in the pathophysiology of post-hemorrhagic hydrocephalus (PHH) in preterm infants. This group has reported alterations in VZ cell junction biology underlying VZ disruption in PHH; specifically, N-cadherin-based adherens junctions are compromised in PHH. Linked to this phenomena are a characteristic neuroinflammatory response, reactive astrocytosis, and resulting periventricular white matter pathology. We now have compelling preliminary data implicating the sheddase A Disintegrin and Metalloproteinase 10 (ADAM10)-mediated cleavage of N-cadherin in VZ disruption. In the current project, this group will test the Central Hypotheses that: 1) ADAM10-mediated cleavage of N-cadherin is a fundamental trigger for VZ disruption and the pathogenesis of PHH; and 2) pharmacological inhibition of ADAM10 will prevent the development and pathophysiology of PHH. This highly innovative proposal will provide the first steps toward defining primary molecular mechanisms that lead to PHH. Grant value: $300k.
Response of the Choroid Plexus to Preterm Hemorrhage — Maria Lehtinen, Boston Children’s Hospital (Partial sponsorship via Team Hydro)
Hydrocephalus refers to a constellation of symptoms in conjunction with enlarged cerebral ventricles and/or elevated intracranial pressures, and a common variant of hydrocephalus occurs in the days and weeks following pediatric intraventricular hemorrhage. Because choroid plexus (ChP) is directly exposed to intraventricular blood products and plays a substantial role in cerebral fluid formation and in coordination of normal brain development, its role in post-hemorrhagic hydrocephalus (PHH) warrants more detailed study. This proposal aims to dissect ChP dysfunction in PHH by evaluating cell-type specific changes in gene expression, protein secretion, and fluid pressures primarily in fetal and juvenile rodents, but also with direct comparisons to cerebrospinal fluid (CSF) samples from human pediatric PHH at the Boston Children’s Hospital. Stemming from the lack of therapeutic options for PHH, the experimental designs will also evaluate and lay the groundwork to identify new therapies for ChP dysfunction in pediatric PHH. Grant value: $25k.
Drug Delivery for Posthemorrhagic Hydrocephalus* — Yun Yung, Scintillon Institution (Partial sponsorship via Team Hydro)
The field of hydrocephalus research has made great strides in understanding the etiologies and downstream sequelae of PHH. Numerous genes and blood-derived factors have been identified in preclinical animal models or clinical studies. A major bottleneck for patients now is identifying and optimizing drug delivery and compounds for non-surgical PHH treatment. Promising approaches include opening blood brain barriers (BBB) using physiochemical methods, as well as permeant, target factor-specific nanoantibodies, which are highly novel and innovative. This group will study these novel approaches for PHH treatment using established fetal and neonatal mouse models induced by blood or lysophosphatidic acid (LPA). This proposal’s goals are to understand how LPA signaling affects brain barriers and erythrocytes to improve drug access into PHH brains. Positive results here could be broadly applicable to various forms of hydrocephalus caused by blood or infection and lead to subsequent NIH applications for basic and clinical studies. Grant value: $25k.
A Molecular Shunt for Curing Hydrocephalus– Linninger Lab, University of Illinois
Hydrocephalus has multiple etiologies with a common symptom of water accumulation in the ventricles. The current treatment standard of fluid shunting via a ventricular catheter has remained largely unchanged over the past 50 years. Because water accumulation and fluid exchange within the brain are poorly understood, there is no cure for hydrocephalus. The astrocyte transmembrane protein, aquaporin-4, has been identified as a critical water transport channel. This project seeks to quantify amount and speed of water flux via AQP4 channels. We will use a novel microfluidic platform especially designed to mimic the in vivo morphology of astrocyte networks to study intracellular water transport. The new insights and data will create the foundation for managing and restoring cerebral water transport by pharmacological intervention. The envisioned pharmacological therapy would eliminate pathological water accumulation via molecular shunting, apt to cure hydrocephalus rather than merely treating its symptoms. Grant value: $50k.
Goal: Develop an in vitro system that measures fluid dynamics and to test drugs that have the potential to improve fluid removal in hydrocephalus.
Therapeutic Modulation of Post-Hemorrhagic Hydrocephalus– McAllister Lab, Wash U.
Few attempts have been made to pharmacologically modulate the pathophysiology of hydrocephalus. However, we recently demonstrated that intraventricular infusions of “Decorin” significantly reduce neuroinflammation and prevent ventriculomegaly if given at the onset of hydrocephalus. Our collaborators have also developed a chemokine antibody that protects against demyelination4. These promising results require further study in a clinically relevant model of hydrocephalus. Neuroinflammatory processes are hallmarks of hydrocephalus, and likely contribute to increases in brain stiffness observed clinically. Brain stiffness can be measured non-invasively with novel magnetic resonance elastography (MRE); this new technique will be used, in combination with other assessments of white matter integrity, cerebrospinal fluid (CSF) biomarkers, intracranial pressure (ICP), and cytopathology to identify specific drug targets and evaluate the effects of drug treatments. Our studies focus on post-hemorrhagic hydrocephalus (PHH) because of its prevalence. Our findings will lead to further development of treatments that could dramatically improve patient outcome. Grant value: $50k.
Goal: Develop a higher model of post-hemorrhage hydrocephalus and determine the efficacy of one drug in stopping disease progression.
Analysis of the role of NFIX in the development of hydrocephalus– Piper Lab, University of Queensland
Our preliminary data have revealed a key role for the transcription factor NFIX in regulating the normal development of neural stem cells within the subventricular zone and of the ependymal cell layer of the lateral ventricles [1]. Moreover, rodents [2] and humans [3] with reduced NFIX expression exhibit abnormal ventricular enlargement, implicating NFIX in the development of hydrocephalus. However, the processes underpinning this remain to be identified. Here we propose to use a suite of innovative techniques, coupled with an Nfix knockout mouse strain, to assess the cellular and molecular mechanisms by which NFIX mediates neural stem cell differentiation and subsequent ependymal cell development. Collectively this study will characterize the role of NFIX in the development of the ventricular system of the brain, and will provide crucial molecular insights into the formation of hydrocephalus, research that will provide the foundation towards developing strategies aimed at ameliorating this debilitating disorder. Grant value: $25k.
Augurin as a novel choroid plexus-derived peptide hormone that regulates CSF formation by controlling epithelial cell homeostasis– Sonia Podvin and Andrew Baird, UCSD
The study hypothesized that the peptide hormone, augurin, is produced and secreted by the choroid plexus epithelium, is a ligand for an unknown receptor and has a critical function in CSF fluid homeostatsis. If proven, researchers predict that augurin can be manipulated pharmacologically to treat hydrocephalus. Grant value: $110k. See here for a Q&A with Dr. Podvin!